The chemical equation representing the reaction of calcium carbonate with hydrochoric acid is:
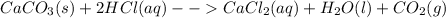
Moles of HCl given =

Moles of
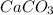 =
=
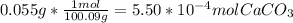
Moles of HCl that would react with
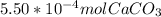 :
:
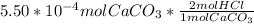 =0.0011mol HCl
=0.0011mol HCl
Moles of HCl that would not be neutralized = 0.0025 mol - 0.0011 mol
= 0.0014 mol HCl