Answer:
493.9°C
Step-by-step explanation:
Given parameters:
Initial pressure =0.815atm
Initial temperature = 265C = 265 + 273 = 538k
Initial volume = 416mL = 0.416L
New volume = 593mL = 0.593L
Unknown:
New temperature =?
Solution:
Since we are dealing with constant pressure condition, we apply charles's law.
It states that the volume of a fixed mass of gas varies directly as its absolute temperature if the pressure is constant.
Mathematically ;
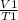 =
=
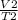
V1 is the initial volume V2 is the new volume
T1 is the initial temperature T2 is the new temperature
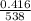 =
=
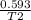
T2 = 766.9K;
In °C = 766.9 - 273 = 493.9°C