Answer : The number of atoms in a mole of any element is,
 (Avogadro's number).
(Avogadro's number).
Explanation :
According to the mole concept, the one mole of an any element contains
 number of atoms.
number of atoms.
For example : In the
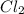 there are two chlorine element or molecule. So, in the
there are two chlorine element or molecule. So, in the
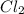 the number of atoms will be,
the number of atoms will be,
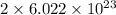 .
.
Hence, the number of atoms in a mole of any element is,
 (Avogadro's number).
(Avogadro's number).