Answer: The correct answer is No.
Step-by-step explanation:
We are given:
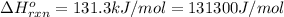 (Conversion factor: 1 kJ = 1000)
(Conversion factor: 1 kJ = 1000)
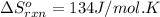
Temperature of the reaction = 298 K
To calculate the standard Gibbs's free energy of the reaction, we use the equation:
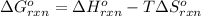
Putting values in above equation, we get:
![\Delta G^o_(rxn)=131300J/mol-[(298K)* (134J/mol.K)]=91368J/mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/x67somynu99tb22oa5u8zxas2f77yqmta1.png)
For the reaction to be spontaneous, the Gibbs free energy of the reaction must come out to be negative. But, from the above calculation, the Gibbs free energy of the reaction is positive. Thus, the reaction is non-spontaneous.
Hence, the correct answer is No.