Answer:
n = A – Z
Explanation:
The general symbol for an atom of an element X is
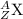 , where
, where
Z = the atomic number = the number of protons (p) and
A = the mass number = the number of protons + the number of neutrons (n)
A = p + n
Subtract Z from A. Then,
A - Z = p + n – p = n
or
n = A - Z