Answer:- 40.7 moles of H
Solution:- It asks to calculate the number of moles of H in 3.7 moles of
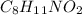 . Looking at the molecular formula of the compound, there are 11 H in it. It means 1 mol of the compound
. Looking at the molecular formula of the compound, there are 11 H in it. It means 1 mol of the compound
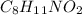 has 11 moles of H. 3.7 moles of this compound would have how many moles of H? It could easily be figured out if we multiply these two numbers and the set up could be shown as:
has 11 moles of H. 3.7 moles of this compound would have how many moles of H? It could easily be figured out if we multiply these two numbers and the set up could be shown as:
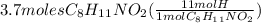
= 40.7 moles H
So, the given compound has 40.7 moles of H in it's 3.7 moles.