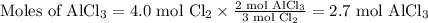Answer:
a) 2.7 mol
Step-by-step explanation.:
2Al + 3Cl₂ ⟶ 2AlCl₃
We want to convert moles of Cl₂ to moles of AlCl₃.
Choose the conversion factor ( the correct molar ratio)
It is or either
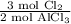 or
or
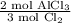
We choose the one with the desired units (mol AlCl₃) on top.