Answer:
B. Atomic number from mass number
Step-by-step explanation:
The general form for an atomic symbol of an element X is
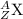
where
Z = the atomic number = the number of protons = p
A = the mass number =the number of protons plus neutrons =p + n
So, to get the number of neutrons, you take
A – Z = (p + n) – p = n
In words,
Mass number – atomic number = number of protons