Answer : 42.3 ml of a 0.266 M
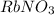 solution are required.
solution are required.
Solution : Given,
Molarity of
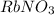 solution 1 = 0.266 M
solution 1 = 0.266 M
Molarity of
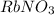 solution 2 = 0.075 M
solution 2 = 0.075 M
Volume of
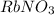 solution 2 = 150 ml = 0.150 L (1 L = 1000 ml)
solution 2 = 150 ml = 0.150 L (1 L = 1000 ml)
Formula used :
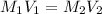
where,
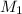 = Molarity of
= Molarity of
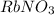 solution 1
solution 1
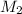 = Molarity of
= Molarity of
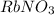 solution 2
solution 2
 = Volume of
= Volume of
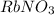 solution 1
solution 1
 = Volume of
= Volume of
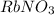 solution 2
solution 2
Now put all the given values in above formula, we get
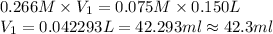 (1 L = 1000 ml)
(1 L = 1000 ml)
Therefore, 42.3 ml of a 0.266 M
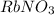 solution are required.
solution are required.