Answer: A reversible reaction is a reaction that takes place in back and front directions. If the reaction were to reach equilibrium, the rate of forward direction would be equal to that of the reverse reaction.
Step-by-step explanation:
Reversible reactions :
These are the reaction in which reactants reacts to give product and products reacts to give reactants as a product in return.
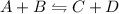
- In above equation, 'A' and 'B' are reacting together to give 'C' and 'D', as products and vice-versa.
- When the above reaction reaches equilibrium the rate of forward and backward reaction becomes equal.