Explanation: An element is represented in the form of
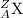 where,
where,
X = Symbol of the element
A = Atomic Mass of the element X
Z = Atomic Number of element X
Hence, For the element Justwondoricium,
Symbol = Jw
Atomic number = 120
Atomic Mass = 224
Now, Atomic number = Number of electrons = Number of Protons
Number of electrons = 120
Number of Protons = 120
and Atomic Mass = Number of neutrons + Number of protons
Number of neutrons can be calculated as we know atomic mass and number of neutrons, Putting the numbers we get
Number of neutrons = 224 - 120 = 104
The nearest noble gas to the element having atomic number 120 is Oganesson (Og), which has an atomic number of 118, so the next two electrons will be filled in the 8s orbital.
Electronic Configuration of Jw is
![[Og]8s^2](https://img.qammunity.org/2019/formulas/chemistry/middle-school/vm6sz4xxn8f8xf4m9hvvipw2w20xyfu5k5.png)
This electronic configuration lets us know about the location of the element in periodic table.
As the electron is entering the 8th shell, it belongs to the 8th period and as the last electron enters the s-orbital, it belongs to the S-block of the periodic table.
When the s-electrons are 1, it belongs to Group 1.
When the s-electrons are 2, it belongs to Group 2.
As this element has 2 s-electrons, it belongs to the Group 2.