Answer: The correct answer is Option 1.
Step-by-step explanation:
A double displacement reaction is defined as the reaction in which exchange of ions takes place. The general equation for the reaction follows:
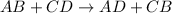
During this reaction, one of the product formed is always a salt.
A gas is formed in a single displacement reaction and not in double displacement reaction.
A precipitate is formed in some of the double displacement reaction.
Water is formed in neutralization reaction when an acid reacts with a base to produce a salt and water.
Hence, the correct answer is Option 1.