Answer: The number of moles of sugar that is used are 0.055 moles.
Step-by-step explanation:
We are given:
Mass of 1 mole of sugar = 342 g
Mass of sugar used = 19 g
So, by applying unitary method, we get:
342 grams of sugar is making 1 mole
So, 19 grams of sugar will make =
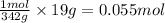
Hence, the number of moles of sugar that is used are 0.055 moles.