Reaction of option c produces precipitate.
Rhodium on reacting with potassium phosphate produces rhodium phosphate which remain in solution due to low lattice energy for rhodium phosphate.
Niobium on reacting with lithium carbonate produces niobium carbonate and it will remain in aqueous form.
Cobalt on reacting with zinc nitrate produces cobalt nitrate. This, Co(NO3 )2 is insoluble precipitate and settles at bottom whereas zinc ion will remain in solution as follows:
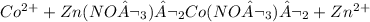
Potassium ion on reacting with sodium sulfide produces potassium sulfide which remain in solution