The reaction is a synthesis…

… because two substances are combining to make one other substance.
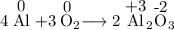
It is also a reduction-oxidation reaction because the oxidation number of Al increases from 0 to +3 and the oxidation number of O decreases from 0 to -2.