Answer : The average atomic mass of an element X is, 63.546 amu
Solution : Given,
Mass of isotope X-63 = 62.9296 amu
% abundance of isotope X-63 = 69.15% = 0.6915
Mass of isotope X-64 = 64.9278 amu
% abundance of isotope X-64 = 30.85% = 0.3085
Formula used for average atomic mass of an element X :
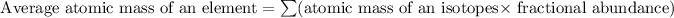
![\text{ Average atomic mass of an element X}=\sum[(62.9296*0.6915)+(64.9278* 0.3085)]](https://img.qammunity.org/2019/formulas/chemistry/college/teibkfpaos5me6qfaayy3ai8qkv0jvl7ip.png)
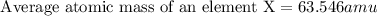
Therefore, the average atomic mass of an element X is, 63.546 amu