Answer:- B. 0.343
Solution:- Hot metal is added to water so the heat is gained by water and lost by the metal. We assume no heat is lost to the surroundings, so the heat lost by metal is totally used to raise the temperature of water.
First, we will calculate the heat gained by water using the formula:
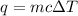
where, q is the heat energy, m is the mass, c is specific heat and delta T is change in temperature.
For water:
m = 8.4 g
c =
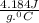
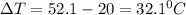
Let's plug in the values and calculate q for water:
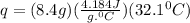
= 1128.17 J
Same amount of heat is lost by the metal. Mass of metal is 68.6 g.
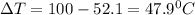
let's plug in the values in the same formula and calculate the specific heat of metal:
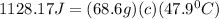
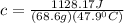
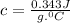
So, the right choice is B.0.343.