The correct answer is:
negative
In fact, the first law of thermodynamics is generally stated as follows:
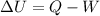
where
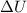 is the variation of internal energy of a system
is the variation of internal energy of a system
Q is the heat absorbed by the system from the surroundings
W is the work done by the system on the surroundings
Following this definition, Q is positive if the heat is absorbed by the system, while it is negative if the heat is released by the system (and therefore, absorbed by the surroundings), because in this case the internal energy of the system decreases.