Answer is 63.04amu.
Step-by-step explanation: We are given in the question that an element has two main isotopes 1 and 2.
Mass Number of isotope 1 = 62 amu
Mass Number of 2 isotope = 65 amu
% abundance of isotope 1 = 66%
Fractional abundance of isotope 1 = 0.66
Total fractional abundance = 1
Fractional abundance of isotope 2 = (1-0.66)
= 0.34
For calculating he atomic mass of element, we use
![\text{atomic mass of element}=\left [(\text{fractional abundance of isotope 1})* (\text {atomic mass of isotope 1)} \right ]+\left [(\text{fractional abundance of isotope 2})* (\text {atomic mass of isotope 2}) \right ]+...](https://img.qammunity.org/2019/formulas/chemistry/middle-school/9jxbfzqkjpzppaz6ulexfhfsku1sw9q88b.png)
We have only 2 isotopes for an element, so the formula stops at isotope 2 only.
By putting the values in the above equation, we get
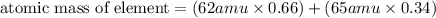
Atomic mass of element = 63.04amu