Answer: He could add a base to the pool to neutralize the acid.
Step-by-step explanation:
Chlorine is used to disinfect the pools as it produces hypochlorous acid in water. This hypochlorous acid is unstable and gives hydrochloric acid and nascent oxygen which is used to disinfect.

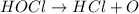
The excess acid is neutralized by adding base which produces salt and water and thus decrease the acidity.
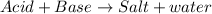
Adding more acid would increase the acidity further.