The balanced chemical reaction between hydrogen and oxygen to form water is given as:
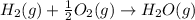
From the balanced chemical equation it is clear that one mole of hydrogen reacts to give 1 mole of water.
Since, number of moles =
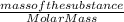
So, number of moles of hydrogen =
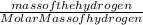
Mass of hydrogen =
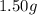 (given)
(given)
Molar mass of hydrogen,

Substituting the values:
Number of moles of hydrogen =
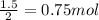
Number of moles of water formed on reacting with
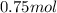 of hydrogen is:
of hydrogen is:
Number of moles of water =
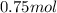
Mass of water can be calculated by:
Number of moles of water =
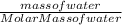
Molar mass of water =
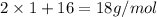
Substituting the values:

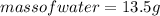
Hence, the mass of water vapor forms is
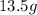 .
.