There are three critical constants namely, critical temperature, critical pressure and critical volume.
Critical temperature is defined as temperature of gas below which the increase in pressure cause liquefaction of gas and above that liquefaction of gas do not take place.
Critical pressure is defined as pressure needed to liquefy a gas at critical temperature. Volume of 1 mol of gas at critical pressure and temperature is known as critical volume.
Critical temperature can be calculated as follows:
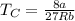
Putting the value,
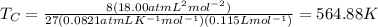
Thus, critical temperature is 564.88 K.
Critical pressure is calculated as follows:
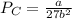
Putting the values,
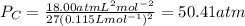
Therefore, critical pressure is 50.41 atm.
Now, calculate critical volume as follows:
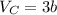
Putting the values,
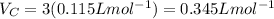
Therefore, for 1 mol critical volume is 0.345 L.