Neutralization reaction is defined as reaction between an acid and a base to form salt and water.
The general reaction is as follows:
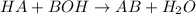
Here, HA is acid, BOH is base and AB is salt formed from corresponding conjugate base and acid.
According to general reaction, the neutralization reaction of HBr and KOH is as follows:

Here, HBr is a strong acid, KOH is a strong base and KBr is neutral salt.
In the above reaction, all the constituents on left hand side of the reaction arrow are equal to the constituents on right hand side thus, the neutralization reaction is balanced.