The mass sample of nicotine combusted = 2.625 mg (given)
Mass of
 produced = 7.1210 mg (given)
produced = 7.1210 mg (given)
Mass of
 produced = 2.042 mg (given)
produced = 2.042 mg (given)
Molar mass of
 = 44 g/mol
= 44 g/mol
Molar mass of
 = 18 g/mol
= 18 g/mol
Percentage of Carbon =
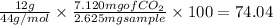 %
%
Percentage of hydrogen =
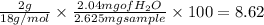 %
%
Now, for percentage of nitrogen =
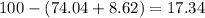 %
%
Calculating the moles of each element:
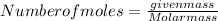
- For
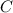
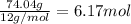
- For
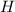
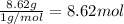
- For
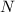
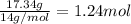
Dividing with the smallest mole value to calculate the molar ratio of each element:
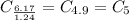
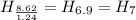
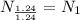
Hence, the empirical formula for nicotine is
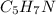 .
.