We are supposed to compare the absolute value of freezing point of chlorine and nitrogen.
Since we know that freezing point of Nitrogen is
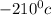 and freezing point of chlorine is
and freezing point of chlorine is
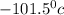 .
.
We know that when we take absolute value of a negative number it makes that number positive.
Now let us take the absolute values of freezing points of chlorine and nitrogen.
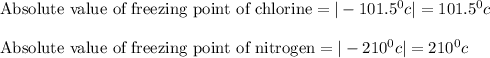
Therefore, we can see that absolute value of freezing point of nitrogen is greater than absolute value of freezing point of chlorine.