The compound responsible for the characteristic smell of garlic is allicin, that is having molecular formula C₆H₁₀OS₂.
Molar mass of C₆H₁₀OS₂=162.26 g mol⁻¹.
number of moles of C₆H₁₀OS₂=1 mol
So mass of C₆H₁₀OS₂ can be calculated as:
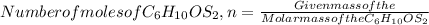
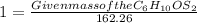
Given mass of the C₆H₁₀OS₂= 162 g