To determine whether the crown was made of pure gold or not, we need to compare the density of gold (19.3 g/mL) to the density of the crown.
The formula for density is
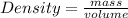
we know the mass of the crown is 714 grams and its volume is 38.3 mL. (Because it displaces 38.3 mL of water)
Using this information, we can plug the numbers into our density formula to solve for the density of the crown.
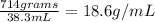
The density of the crown is 18.6 g/mL, thus the crown was not made of pure gold, as that has a density of 19.3 g/mL. In conclusion, the goldsmith tried to screw over the king.