A reaction between acid and base to form water and salt is known as neutralization reaction. It is a double replacement reaction.
The reaction between
 and
and
 will be:
will be:
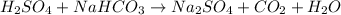
The balanced reaction is:
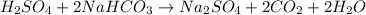
Volume of
 = 28 mL (given)
= 28 mL (given)
Since, 1 L = 1000 mL
So, 28 mL = 0.028 L
Molarity of
 = 5.4 M (given)
= 5.4 M (given)
Molarity =
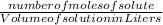 -(1)
-(1)
Substituting the values in equation (1):
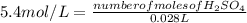
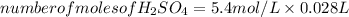
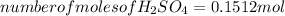
From the balanced reaction between
 and
and
 , 2 moles of
, 2 moles of
 reacts with 1 mole of
reacts with 1 mole of
 .
.
Molar mass of
 = 84.007 g/mol
= 84.007 g/mol
Mass of
 needed:
needed:
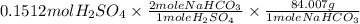 =
=
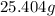
Hence, the required amount of
 is
is
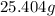 .
.