The given question is incomplete. The complete question is as follows.
What is the mass, in grams, of 28.80 ml of acetone? Acetone (nail polish remover) has a density of 0.7857
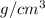 .
.
Step-by-step explanation:
It is known that density is the mass of a substance occupied in per unit volume of the object.
Mathematically, Density =
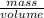
Therefore, putting the given values into the above formula as follows.
Density =
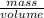
0.7857
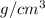 =
=
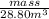 (1 ml = 1
(1 ml = 1
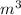 )
)
mass = 22.62 g
Thus, we can conclude that mass of given acetone is 22.62 g.