The mass of piece of sterling silver jewelry is 33.24 g. It contains 92.5% silver Ag by mass. Since, sterling silver is an alloy of Ag-Cu thus, percentage of Cu will be:
% Cu=100-92.5=7.5%
Thus, mass of copper will be:
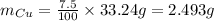
Molar mass of Cu is 63.546 g/mol, thus, number of moles of Cu can be calculated as follows:
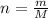
Here, m is mass and M is molar mass.
Putting the values,
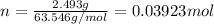
Now, in 1 mole of Cu there are
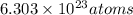 thus, in 0.03923 mol, number of Cu atoms will be:
thus, in 0.03923 mol, number of Cu atoms will be:
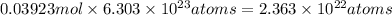
Thus, number of atoms of Cu will be
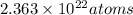 .
.