Answer : The number of electrons present in the 5th shell are, 50.
Explanation :
As we know that the electrons are filled in the electrons shell of an atom from lower energy shells to the higher energy shells. Each shell contains fixed number of electrons that means 1st shell contains 2 electrons, 2nd shell contains 8 (2+6) electrons, 3rd shell contains 18 (2+6+10) electrons and so on.
The general formula of for the 'nth' shell will be :
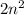 . Where 'n' is the number of shell.
. Where 'n' is the number of shell.
As we are given that the number of shell is, 5th
So, the number of electrons present in 5th shell are =
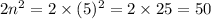
Hence, the number of electrons present in the 5th shell are, 50.