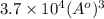Let volumetric size of a water molecule = v
Since, 1 mole of water consists of
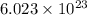 molecules of water.
molecules of water.
Thus, total volume of 1 mole of water =
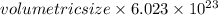
Substitute the value of total volume of 1 mole of water i.e. 22.4 L in above formula.
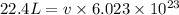
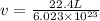
Convert the unit litre to angstrom
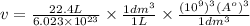
=
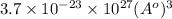
=
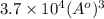
Therefore, the volumetric size of the water molecule is