The method of determining the most common isotope of lead is by determining the average atomic mass. The formula for determining the average atomic mass is:
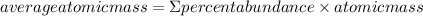
Substituting the values in the formula:
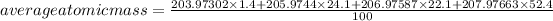
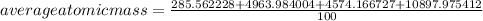
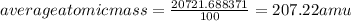
The most common isotope of lead is:
Lead - 207.22 amu
The atomic symbol of lead is
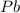 . The atomic number of
. The atomic number of
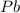 is 82.
is 82.
So, the the most common isotope of lead can be written as:
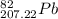 and Lead - 207.22 amu.
and Lead - 207.22 amu.