The reaction of acetic acid with sodium hydroxide is:
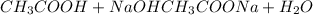
The ratio of A-/HA is calculated as follows:
According to Henderson Hasslebach equation:
![[A-]/[HA] = 10^p^H^-^p^K^_a](https://img.qammunity.org/2019/formulas/chemistry/high-school/bt5koz8kbzoiyl2rqhdrzfstisvpb5keow.png)
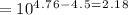
The total concentration of HA and A- = 2.0 L * 0.25 M = 0.5 mol.
![[ A- ]+ [ HA ]= 0.5](https://img.qammunity.org/2019/formulas/chemistry/high-school/potj4raohscx3nkvgi94n1ukglxrqhda8e.png)
![[ A^- ] = 0.5 – [ HA]](https://img.qammunity.org/2019/formulas/chemistry/high-school/lm8yaevs5r1pegp2y8ebumsd4yxdvsjc0e.png)
[ HA] = 0.156 mol and [ A- ]= 0.343 mol
Total of 0.5 moles of acetic acid is required:
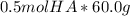
HA = 30.0 g acetic acid
Conversion of 0.343 moles of the acetic acid to acetate can be performed by adding NaOH
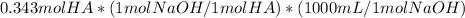
= 343 mL
Thus, 343 mL of 1M NaOH is required