The molecule with same molecular formula but different arrangement of atoms is said to be an isomer.
When 2,2-dimethylbutane reacts with chlorine in the presence of light gives three isomers that is
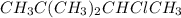 (3-chloro-2,2-dimethylbutane),
(3-chloro-2,2-dimethylbutane),
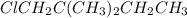 (1-chloro-2,2-dimethylbutane) and
(1-chloro-2,2-dimethylbutane) and
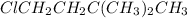 (1-chloro-3,3-dimethylbutane).
(1-chloro-3,3-dimethylbutane).
In above case, the molecular formula of all isomers are same i.e.
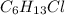 but chlorine is arranged in different positions of carbon. Thus, results isomers.
but chlorine is arranged in different positions of carbon. Thus, results isomers.
The reaction is shown in the image.