The reaction for burning of charcoal or complete combustion is as follows:
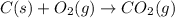
From the above balanced reaction, 1 mole of carbon releases 1 mole of
 gas.
gas.
Converting mass of charcoal into moles as follows:
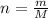
Molar mass of pure carbon is 12 g/mol thus,
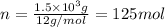
The same moles of
 is released. Converting these moles into mass as follows:
is released. Converting these moles into mass as follows:
m=n×M
Molar mass of
 is 44 g/mol thus,
is 44 g/mol thus,
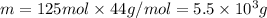
Converting mass into kg,
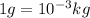
Thus, total mass of gas released is 5.5 kg.