10% solution means=
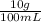
=
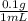
= 100 mg/mL
If the dentist inject 0.3 mL, that is equal to 0.3× 100 mg.
If the dentist inject 0.3 mL, that is equal to 30 mg.
So, as anesthetic procaine hydrochloride is often used to deaden pain during dental surgery. the compound is packaged as a 10.% solution (by mass; d = 1.0 g/ml) in water. When our dentist injects 0.30 ml of the solution, 30 mg of procaine hydrochloride (in milligrams) is injected.