Answer:
Specific gravity of the solution is 1.18
Step-by-step explanation:
It is given that,
The density of a solution, d₁ = 1.18 g/ml
We have to find the specific gravity of the solution. Specific gravity is given by the ratio of density of substance to the density of water. Mathematically, it can be written as :
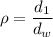
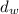 is the density of water and
is the density of water and
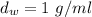
It gives,
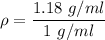
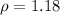
Specific gravity is an unit less quantity. So, the specific gravity of the solution is 1.18. Hence, this is the required solution.