Answer:- There are
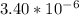 moles.
moles.
Solution:- It is a unit conversion problem where we are asked to convert mg of aspartame to moles. Aspartame is
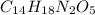 and it's molar mass is 294.31 grams per mole.
and it's molar mass is 294.31 grams per mole.
mg are converted to grams and then the grams are converted to moles as:
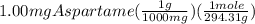
=
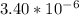 moles of aspartame
moles of aspartame
So, there would be
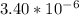 moles of aspartame in 1.00 mg of it.
moles of aspartame in 1.00 mg of it.