Electrophiles are reagents attracted to electrons.
Electrophiles tend to be electron-deficient and carry partial positive charges. They are attracted to species with lone pairs of electrons. For example, protons
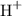 have no electrons and tend to share ones with other species, hence behaving as electrophiles in aqueous reactions. In the reaction between
have no electrons and tend to share ones with other species, hence behaving as electrophiles in aqueous reactions. In the reaction between
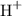 and ammonia
and ammonia
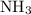 , protons would be attracted to lone electron pairs on nitrogen atoms in ammonia molecules, which carry partial positive charges.
, protons would be attracted to lone electron pairs on nitrogen atoms in ammonia molecules, which carry partial positive charges.
The Lewis Acid-base theory define Acids as species that accept electron pairs in a particular acid-base reaction. Electrophiles, by definition, tend to accept electrons. Lewis acids thus behaves as electrophiles in acid-base reactions. In the previous example,
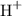 demonstrates acidic behavior and can be inferred as an electrophile.
demonstrates acidic behavior and can be inferred as an electrophile.