Answer : The volume of the gas sample is 0.00192 L.
Explanation :
The density of a substance is defined as the mass per unit volume.
The formula for density can be written as
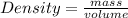
Step 1 : Convert mass from mg to g.
The density of the sample is given as 1.17 g/L
The mass of the sample is 2.25 mg.
Since density has the unit "g", we need to convert the mass from mg to g.
The conversion factor for g to mg is , 1 g = 1000 mg.
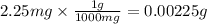
Step 2 : Use density formula.
The density formula can be rearranged to solve for volume as follows.
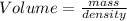
Mass = 0.00225 g
Density = 1.17 g/L
Let us plug in these values in the above formula.
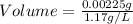
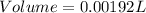
The volume of the gas sample is 0.00192 L or 1.92 mL