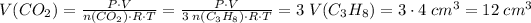A-
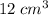
From coefficients in the equation, for each mole of propane combusted, three moles of carbon dioxide molecules are produced. Thus
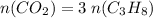
The question implied that the temperature and pressure on both carbon dioxide
 and propane are those under room conditions- so these values are supposed to be interchangeable; thus by the ideal gas equation
and propane are those under room conditions- so these values are supposed to be interchangeable; thus by the ideal gas equation