Answer:- Mass of given moles of NaCl is 8.2 g.
Solution:- It is a unit conversion problem from moles to grams. We have been given with 0.14 moles of NaCl and asked to calculate the grams.
For moles to grams conversion we multiply the given moles by the molar mass. Molar mass of NaCl is given as 58.44 grams per mol.
mass = moles* molar mass
mass =
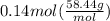
mass = 8.2 g
So, the mass of given moles of NaCl is 8.2 g.