Answer: -
79.11%
Explanation: -
Actual mass of CO₂ = 60.5 g
Molar mass of CO₂ = 12 x 1 + 16 x 2 = 44 g / mol
Actual number of moles of CO₂ =
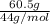
= 1.375 mol of CO₂
Mass of C₂H₂ = 22.6 g
Molar mass of C₂H₂ = 12 x 2 + 1 x 2
= 26 g / mol
Number of moles of C₂H₂ =
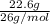
= 0.869 mol
The balanced chemical equation for the reaction is
2 C₂H₂ + 5 O₂ → 4 CO₂ + 2 H₂O
From the equation we see that
2 mol of C₂H₂ gives 4 mol of CO₂
0.869 mol of C₂H₂ gives
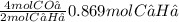
= 1.738 mol of CO₂
Percentage yield =
![\frac{1.375 mol of CO₂{1.738 mol of CO₂}]() x 100
x 100
=79.11%