Answer: -
29.96 moles of KBr
Explanation: -
The balanced chemical equation for the formation of KBr from BaBr₂ is
BaBr₂ + K₂SO₄ 2KBr + BaSO₄
From the balanced chemical equation we see
1 mole of BaBr₂ gives 2 moles of KBr
14.98 moles of BaBr₂ gives
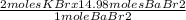 moles of KBr
moles of KBr
= 29.96 moles of KBr.