Answer: [X] increases.
Explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For the given equation:
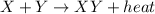
This is a type of Exothermic reaction because heat is released in the reaction.
If the temperature is increased, so according to the Le-Chatlier's principle , the equilibrium will shift in the direction where decrease in temperature occurs. As, this is an exothermic reaction, reverse reaction will decrease the temperature. Hence, the equilibrium will shift in the backward direction and thus [X] increases.