Answer:
7.50 g/ml
Step-by-step explanation:
Density is the ratio of mass to volume
Density =
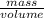
Given,
Mass of the substance = 693.8 g
Volume of the substance = 92.5 mL
Density =
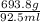 = 7.50 g/ml
= 7.50 g/ml
693.8 has 4 significant figures and 92.5 has 3 significant figures. Hence the answer will have the least number of significant figures i.e. 3. So the correct answer is 7.50 g/ml.