Hello!
The energy required to melt 13 kg of ice at 273k is 5040000 J
Step-by-step explanation:
For solving this exercise, we are going to use the equation that relates the energy in Joules (E) with the Specific Latent Heat of Fusion (l) and the mass of ice (m):
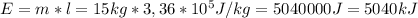
Have a nice day!