For this problem, we are going to be using multiplication and cancelling units. We need to first find the molar weight of mercury, found on the periodic table as 200.59g/mol.
So then we set up the equation:
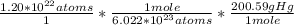
Then we multiply straight across to find the mass in grams:

So now we know that
the mass of this sample is 3.99g Hg.