Answer: dissociates in water, is an electrolyte, is a salt.
Explanation: KCl is an ionic compound formed by the combination of a metal (
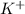 )and a non metal (
)and a non metal (
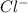 ).
).
Being a polar compound, it dissolves in water and dissociates into
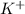 cations and
cations and
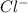 anions. The generation of ions help in the conduction of electricity and thus act as a electrolyte.
anions. The generation of ions help in the conduction of electricity and thus act as a electrolyte.
It is a salt formed by the neutralization of acid with a base.
